
(Professor Haeshin Lee from the Department of Chemistry)
Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
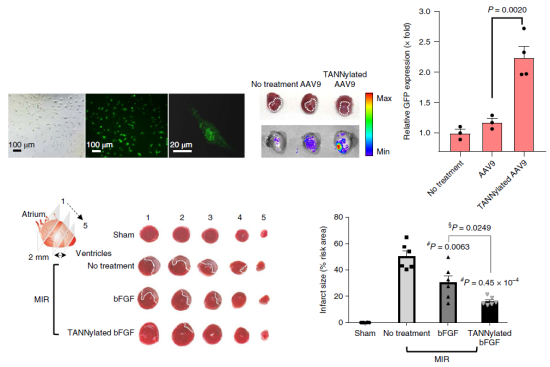
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
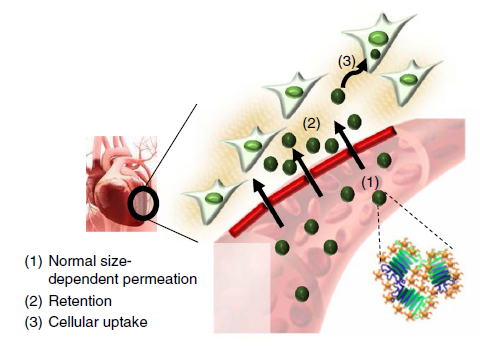
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
SOURCE: KAIST


