
(from left: PhD candidate Soohyun Kim, Professor Hee-Tak Kim and PhD candidate Junghoon Choi)
There has been growing demand for large-scale storage for energy produced from renewable energy sources in an efficient and stable way. To meet this demand, a KAIST research team developed a new vanadium redox-flow battery (VRFB) with 15 times greater capacity retention and five times longer durability. This VRFB battery can be an excellent candidate for a large-scale rechargeable battery with no risk of explosion.
The VRFB has received much attention for its high efficiency and reliability with the absence of cross-contamination. However, it has the limitation of having insufficient charge and discharge efficiency and a low capacity retention rate because its perfluorinated membrane is very permeable to any active materials. To minimize energy loss, it needs a membrane that has low vanadium ion permeability and high ion conductivity. Hence, there was an attempt to incorporate a hydrocarbon membrane that has low cost and high ion selectivity but it turned out that the VO₂+ caused chemical degradation, which led to shortening the battery life drastically.
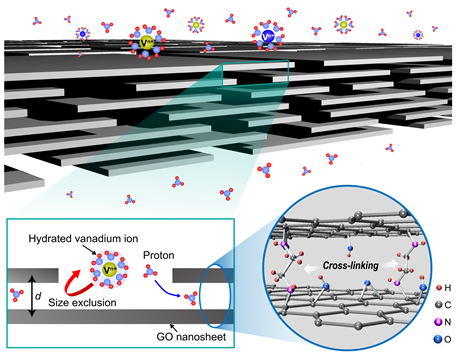
To develop a membrane with pore sizes smaller than the hydrated size of vanadium ions yet larger than that of the protons, a research team co-led by Professor Hee-Tae Jung and Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering implemented a graphene-oxide framework (GOF) membrane by cross-linking graphene oxide nanosheets. They believed that GOF, having strong ion selectivity, would be a good candidate for the membrane component for the VRFB. The interlayer spacing between the GO sheets limited moisture expansion and provided selective ion permeation.
The GOF membrane increased the capacity retention of the VRFB, which showed a 15 times higher rate than that of perfluorinated membranes. Its cycling stability was also enhanced up to five times, compared to conventional hydrocarbon membranes.
These pore-sized-tuned graphene oxide frameworks will allow pore-sized tuning of membranes and will be applicable to electrochemical systems that utilize ions of various sizes, such as rechargeable batteries and sensors.
Professor Kim said, “Developing a membrane that prevents the mixing of positive and negative active materials has been a chronic issue in the field of redox-flow batteries. Through this research, we showed that nanotechnology can prevent this crossover issue and membrane degradation. I believe that this technology can be applied to various rechargeable batteries requiring large-scale storage.”
This research was published in Nano Letters on May 3.
Figure 1. Electrochemical performances of the VRFBs with Nafion 115, SPAES (sulfonated poly), and GOF/SPAES: discharge capacity
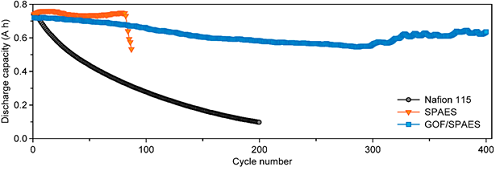
Figure 2. Schematic of the selective ion transfer of hydrated vanadium ions and protons in the GOF membrane and the molecular structure of the GOF membrane, showing that the GO nanosheets are cross-linked with EDA (ethylenediamine)
Source: KAIST


